We are pleased to inform you that BIT Group USA, Inc. will be consolidated into one location in Irvine/ CA. This will allow us to realize synergies and to expand our capabilities to better serve your growing needs.
News
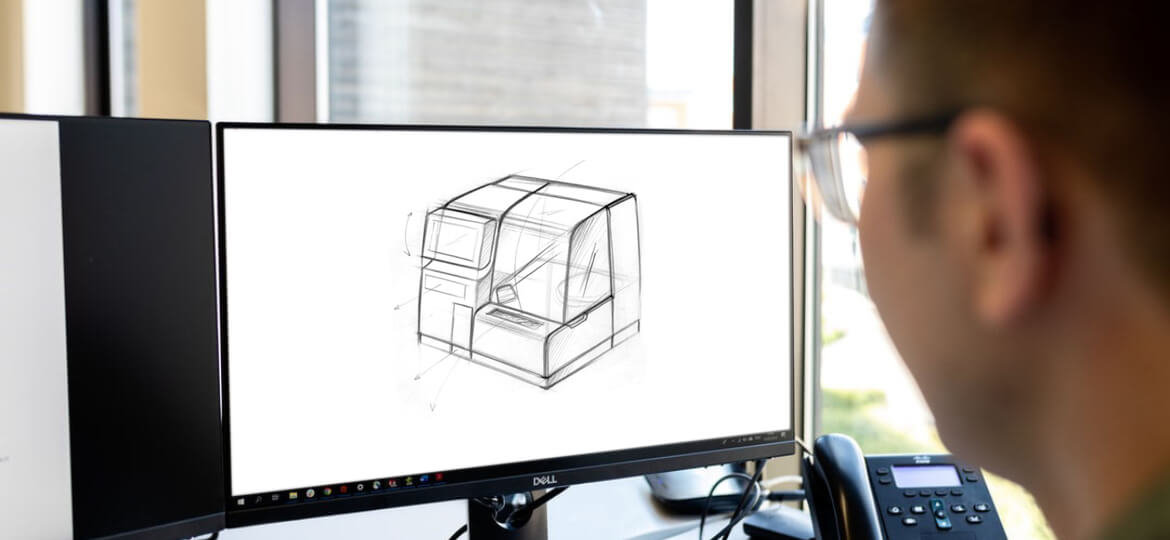
In-Vitro Diagnostics (IVD) instruments are complex systems that require a transdisciplinary engineering approach that includes all disciplines, such as mechanical, electrical, fluidical, optical, software, as well as biological and chemical components.
Design for Manufacturing (DFM) is the process of designing parts, components, or products for ease of manufacturing with an end goal of making a better product in a cost-efficient way.
A note from Messer (BIT’s parent company) on their activities in response to the Coronavirus
Contract manufacturing is in BIT’s DNA. Founded in 1976, BIT began the journey as a medical device contract manufacturer in Frankfurt, Germany.
With just over 25 years’ experience in molecular automation development and manufacturing, BIT is excited to be exhibiting at AMP’s 25th-anniversary meeting.
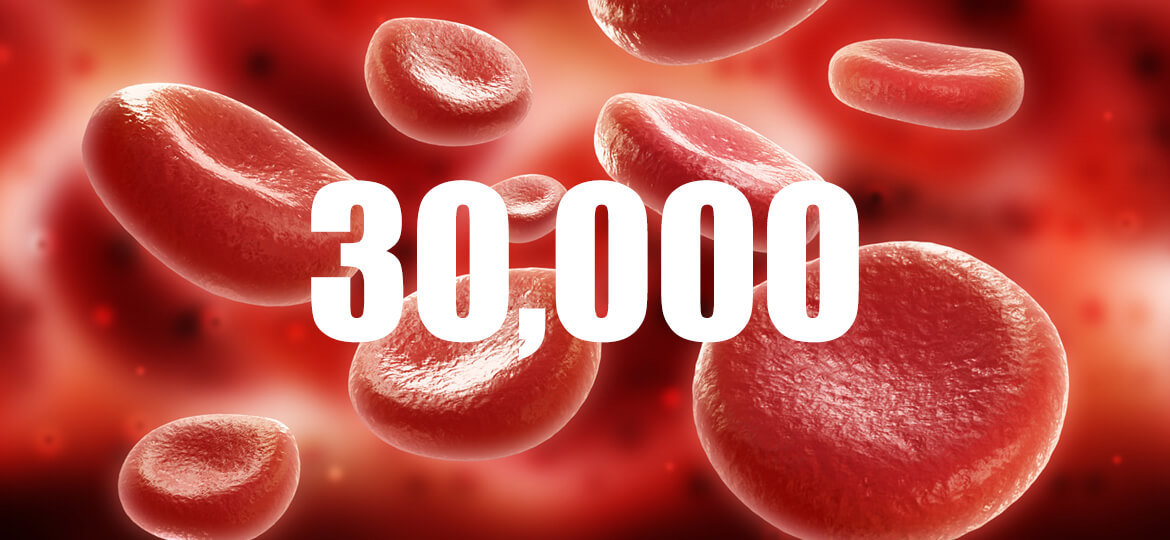
Thanks to 25 years of leadership experience in hematology innovation, BIT has delivered more than 30,000 hematology instruments to its diagnostics clients.
BIT’s mission is to research, develop and manufacture innovative medical diagnostic devices for our partners to improve and save lives.
At the 2019 AACC Expo, BIT will proudly present their latest exciting cutting-edge instrumentation and technologies that are helping to revolutionize the IVD Industry.
Are you ready to transition your manual test to automation but can’t find a clear direction moving forward?
Let BIT help you solve your automation issues by leveraging 40 plus years of market experience and full solution offering.